Vascular malformations of the
neonatal brain
Pierre Lasjaunias, Georges Rodesch and Hortensia Alvarez
Chapter Contents
- Introduction
- Prenatally diagnosed lesions
- Familial lesions
- Sporadic lesions
- Clinical presentation and management
- Conclusions
- Summary
- References
Introduction
Vascular ‘malformations’ include anatomical, pathophysiological and clinical entities as diverse as cerebral arteriovenous shunts, dural meningeal arteriovenous shunts, and arterial aneurysms. The differences between these disorders is now well recognized, but there are additional differences within each group: within the group of cerebral arteriovenous shunts, for instance, age of onset of initial signs. This is best illustrated by comparing aneurysmal malformations of the vein of Galen with true cerebral (or pial) malformations (see below). The distinction is both anatomical and clinical and suggests that the underlying mechanisms may differ2. A similar difference occurs within the group of dural lesions. Although they have been well defined in adults because of their prevalence, little is known about them in children because of their rarity. Venous sinuses of the cranial cavity are formed within the dura during the fetal and postnatal periods, at which times malformations may occur. Venous sinus malformations are found in infants but are never found in adults who present with dural lesions. Aneurysms are also a heterogeneous group. They are difficult to manage in children, because they are so seldom encountered, and because the etiology is different from aneurysms found in adults.
The concept conveyed by the word ‘congenital’ is that an individual with a vascular malformation was born with the lesion, which has remained basically unchanged. Consequently the clinical goals that we have set are early diagnosis and early treatment, whether it be preventive or curative, in an effort to prevent or cure the disorder’s natural history which, we suppose, begins at birth2, 3.
However, there is much evidence to show that it is not that simple. In Rendu–Osler’s disease (regardless of the presence of cerebral arteriovenous lesions), polycystic kidney disease (with arterial aneurysms), fibromuscular dysplasia, or type 1 neurofibromatosis, no morphological lesions can be detected in children belonging to affected families. The first signs do not usually appear until early adulthood. The best-known example of this is polycystic kidney disease, in which the first aneurysms are not diagnosed until approximately 30 years of age, ‘only’ 10 years earlier than in unaffected families. This suggests that familial and genetic predisposition are not enough in themselves to cause aneurysms, but that other intrinsic or extrinsic phenomena (mutation, traumatic triggering factor such as a virus) must occur to morphologically reveal the disease. Likewise, what we consider to be acquired lesions may actually be malformations that are systematically revealed in the same conditions by a factor that has been identified but that does not truly cause the disorder.
Hence the concept of malformation shifts from a morphological to a biological entity. Congenital vascular malformations date from the first stages of development, irrespective of when they are detected (Table 12.1).
Table 12.1 So-called congenital or malformative intracranial vascular lesions
- Familial (seldom symptomatic at pediatric age)
- Arterial aneurysm (polycystic kidney disease, Chr 16, Chr 6)
Pial arteriovenous shunts (Rendu-Osler-Weber, Chr Figs. Chr 9)
(the cerebral phenotype is not transmitted: in children lesions are usually high-flow multifocal AV fistulae)
Cavernomas (Chr 7); often multiple located in the brain and even the cord - Sporadic or primitive
- Vein of Galen aneurysmal malformations (30% diagnosed in utero)
Pial arteriovenous shunts (cerebral), isolated, multiple, systematized (Bonnet Dechaume et Blanc or Wyburn-Mason syndromes)
Dural sinus malformation: torcular, transverse sinus, sagittal sinus, sigmoid sinus (50% diagnosed in utero)
Single cavernoma
Sturge-Weber syndrome - Acquired or secondary
- Dural arteriovenous shunt (juvenile and adult types, sigmoid and transverse sinus, cavernous plexus)
- Vascular anomalies
- Developmental venous anomaly
Sinus pericranii
Embryonic vessel persistence
Chr = chromosome

Fig. 12.1 Typical appearance of a vein of Galen aneurysmal malformation on fetal MRI. T1 weighted images. The lesion is seen as low signal intensity (arrow).

Fig. 12.2 A 1-day-old baby with severe cardiac failure. Infant with a vein of Galen aneurysmal malformation. CT scan shows the lesion as a rounded mid-line lesion with increased attenuation (arrow). There is, however, diffuse bilateral severe brain damage (arrowheads), which represents a contraindication to treatment.
The brain’s specific vulnerability during the perinatal period is such that most arteriovenous malformations diagnosed in children are associated with structural alterations. During his or her first years, a child is not a miniature adult, nor are his or her pathologies those of an adult’s. Conversely there is little chance that lesions discovered in adults were present during infancy or childhood without having left any detectable marks2.

Fig. 12.3 Chest X-ray of a neonate presenting with acute heart failure due to a high-flow arteriovenous brain malformation. Cardiac enlargement due to cardiac overload is apparent, with predominantly right-sided enlargement of the cardiac silhouette.
Every structural and hemodynamic change occurring in the cardiovascular system acts as a shear stress on blood vessels. This stress influences the modeling and remodeling of vessels. A persisting active arteriovenous communication may also cause hypertrophy of the right ventricle and atrium (Fig. 12.3). It may also, depending on its severity, result in respiratory, renal or hepatic failure, and peripheral arterial diastolic steal. Note, however, that adult carriers of cerebral arteriovenous shunts never have a history of perinatal heart failure, regardless of the flow rate within the lesion.
In infants, cerebrospinal fluid is reabsorbed via the cortical and deep cerebral veins, as the Pacchioni granulations in the sagittal sinus are still immature. This explains why venous hypertension (induced by arteriovenous shunts) prevents CSF reabsorption and causes macrocrania, because of excess fluid in the brain (Fig. 12.4 and Fig. 12.5). When this pressure imbalance is marked, hydrocephalus occurs, as a way of alleviating the stress induced by the abnormal venous hemodynamics. Once again, note that adult carriers of arteriovenous shunts rarely have macrocrania.
< prev | top | contents | next >
Table 12.2 Clinical presentation
- In utero
- Congestive cardiac failure (heart rate >200/min, ventricular extrasystoles, tricuspid insufficiency); macrocrania, ventriculomegaly
- Neonate
- Systemic disorders: congestive cardiac failure, multiorgan failure,consumption coagulopathies
Intracranial hemorrhage (hematoma, venous infarct, subarachnoid hemorrhage, convulsions) - Infant
- Hydrovenous disorders: macrocrania, hydrocephalus, convulsions
Intracranial hemorrhage, (hematoma, venous infarct, subarachnoid hemorrhage) - Child
- Intracranial hemorrhage (hematoma, venous infarct, subarachnoid hemorrhage), convulsions
Progressive neurological deficits
Headaches


Fig. 12.4 Example of hydrodynamic disorders at the supratentorial level in an infant with a vein of Galen aneurysmal malformation. Note the ventriculomegaly (a) that resolves after partial embolization of the lesion (b).

Fig. 12.5 Example of hydrodynamic disorders at the infratentorial level in an infant with a vein of Galen aneurysmal malformation. Note the tonsilar prolapse giving rise to a pseudo-Chiari appearance (large arrow). The brain stem is surrounded by tortuous veins (double small arrows), that represent the diversion of blood flow because of bilateral sigmoid thrombosis.

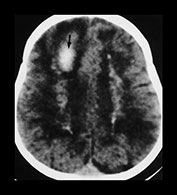
Fig. 12.6 Three-week old neonate presenting with seizures and progressive macrocrania. On T2 weighted MRI (a), although the vein of Galen is dilated (double arrow), the bilobed aspect of the venous ectasia is not consistent with a classical aneurysmal malformation of the vein of Galen. The lesion was diagnosed as a pial arteriovenous fistula draining into the deep venous system (arrow) ending in the vein of Galen that dilated because of the high flow. Urgent embolization to reduce the hydrovenous effects of the shunt was recommended, but refused by the parents. One month later a control CT scan was performed (b) which showed massive brain destruction (‘melting brain syndrome’).
The venous hemodynamic balance required for synaptogenesis, myelogenesis and the maturation of granulations is essential for normal brain growth. Arteriovenous shunts that are active from birth are associated with rapid brain atrophy, most likely due to apoptosis, and cause the brain to ‘melt’ (Figs. (Fig. 12.2) and (Fig. 12.6) ). The disorder is bilateral and symmetrical when venous drainage is located on either side of the deep venous or dural sagittal system; it is focal when drainage is lateral, in the cortical veins of a single hemisphere. Once again, this phenomenon is specific to the pediatric population: focal cerebral atrophy is never detected in adult carriers of arteriovenous shunts. Finally, the deleterious effect of arteriovenous shunts on both myelination and synaptogenesis is associated with neurocognitive impairment in children, while adult carriers of this type of lesion are not usually intellectually impaired.
Thus, we can define congenital vascular lesions as the structural translation of a genetic defect, whether inherited or not, in the arterial or venous system. It can be expressed at any stage of life, when the vascular system is being either modeled or remodeled. It is induced by a secondary event (Table 12.2).
< prev | top | contents | next >
Prenatally diagnosed lesions
As a result, we cannot expect to offer prenatal diagnosis for every type of vascular lesion that belongs to the category entitled ‘malformation’, since a number of them are not expressed until adulthood, while others may even be caused by a postnatal event (Table 12.3). From a practical point of view, two types of malformations are detected before birth: aneurysmal malformations of the vein of Galen and defective dural sinuses.
Table 12.3 Vascular lesions per age group in order of frequency
- In utero
- Aneurysmal malformations of the vein of Galen
- Dural sinus malformation
- Neonates
- Aneurysmal malformation of the vein of Galen
- Dural sinus malformation
- Pial arteriovenous shunt
- Cavernoma
- Arterial aneurysm
- Infants
- Aneurysmal malformation of the vein of Galen
- Dural sinus malformation
- Pial arteriovenous shunt
- Cavernoma
- Arterial aneurysm
- Children
- Pial arteriovenous malformation
- Cavernoma
- Arterial aneurysm
- Dural arteriovenous shunt (juvenile type)
Aneurysmal malformations of vein of Galen1, 4 can be diagnosed as early as the 28th week of pregnancy by ultrasonography or MRI (Fig. 12.1). They appear as a rounded intracranial mass behind the third ventricle in which flow compatible with an arteriovenous shunt can be detected. Associated signs may include heart failure with tachycardia, ventricular extrasystoles, and tricuspid regurgitation. Vein of Galen malformations are rare congenital connections occurring between intracranial vessels. They occur within the first months of development and there is a strong association with persistent venous anomalies such as an absent straight sinus and persistent falcine and occipital sinuses. This does not imply that intrauterine straight sinus thrombosis with subsequent recanalization is responsible for the malformation but represents a time marker for an event that occurred prior to the straight sinus development. Despite their early origins only about half the aneurysmal malformations of vein of Galen are diagnosed antenatally even with good quality ultrasonographic investigations. In utero macrocrania is infrequent and does not imply a poor prognosis. It is usually linked to the volume of the aneurysmal sac rather than to accumulated fluid within the cranial cavity. The natural progression of the disorder is now well established, as are treatment and intervention techniques, which are discussed below.
Malformations of the dural sinuses originate during the fetal period, when sagittal and transverse sinuses differentiate after a normal phase of substantial enlargement around the torcular2. The size of the sinuses spontaneously decreases before birth as they open into a venous system within the dura mater along the edge of the foramen magnum. Malformations that develop at this stage will cause giant venous ‘lakes’ extending on either side of the mid-line. Because of the immaturity of the sinuses, they thrombose spontaneously and cause venous brain infarction and hemorrhage as the process spreads throughout the cerebral venous system. Only a third of dural sinus malformations are diagnosed before birth. The development of the jugular bulbs ends several weeks after birth. Postnatal malformations are due to defective maturation of the jugular bulb, which is due to regression of the embryonic sinus around the occiput. The malformations only affect sigmoid sinuses with secondary arteriovenous communications, are usually easy to treat, and have a good prognosis.
Vein of Galen aneurysmal malformations and malformations of the dural sinuses are the only two true intracranial malformations that we have diagnosed antenatally on ultrasound. Failure to detect pial arteriovenous malformations (AVMs) before birth suggests that they only appear secondarily, after age 2 years, when the brain is more mature and the vulnerable period has finished or that they may be dormant and only manifest clinically later in life. Very rarely pial AVM are diagnosed neonatally but they represent less than 1% of all AVMs seen in our center at Bicêtre, and 5% of those brain AVMs seen in children below 15 years.
< prev | top | contents | next >
Familial lesions
There are no familial disorders that cause AVMs. Although one of the phenotypes of Rendu–Osler disease, which preferentially affects the head, neck, and sometimes the lungs, is associated with cerebral vascular lesions, these lesions are not inherited and seem to follow an independent distribution among families. The AVMs found in children carrying the Rendu–Osler disease all conform to a specific architecture: focal or multifocal fistulae with high flow rates and large venous ectasia. Signs appear later on in life with mental deficiency or more seldom hemorrhage. They are well tolerated for many years. There is no aneurysmal malformation of the vein of Galen in either child or adult carriers of the Rendu–Osler disease. Their flow rate and the lack of cardiac effects suggest that the AVMs do not become active until early childhood, once again after the age of 2years. Murmurs can be heard on skull auscultation, but children fail to complain about them because they are a part of their primary acoustic environment. Diagnosis is made after having established the family history, with epistaxis in parents and other relatives. Mucocutaneous telangiectasia, although characteristic of the disease in adults, is not present at this age.
Cavernomas1, 4, 5 are focal or multifocal venous malformations that may be familial but that rarely occur in children. They are usually small, well-delineated, intraparenchymal lesions that spontaneously appear as dense lesions, which enhance well with contrast, but are angiographically occult. Nevertheless, their diagnosis must be considered when facing relatively well-tolerated intracerebral hemorrhage (or more rarely intraventricular hemorrhage) in infants presenting with convulsions (Fig. 9.20). In familial forms, multiple cavernomas can appear de novo over the course of time. This is a further example of a dormant genetic disorder that requires either a second mutation or an unknown triggering factor to become active.
Familial forms of aneurysm are rare, and mainly found in dominant renal polycystic disease. Recessive forms can nevertheless be found in children. Subarachnoid hemorrhage is usually the initial presenting sign. When these malformations do exist in the pediatric population, they occur mainly in children aged 5 years or more and infants are rarely affected. Familial forms of aneurysm with no known genetic disorder have been reported. However, the ratio of children presenting with intracranial hemorrhage is no higher among these families than in the general population. Consequently, it is difficult to recommend systematic screening of the members of a family; in addition, it is difficult to provide guidelines on the frequency of follow-up examinations. Thus, the mere fact of belonging to a family in which several relatives are affected with aneurysms does not necessarily imply that a child will develop aneurysms during his or her early years, much less during the neonatal period.
< prev | top | contents | next >
Sporadic lesions
Sporadic lesions can be linked to congenital diseases as defined above, with an initial defect that is deciphered secondarily. The structural lesion may be detected or cause clinical signs at any stage of life.
< prev | top | contents | next >
Clinical presentation and management of intracranial arteriovenous shunts
ANEURYSMAL MALFORMATIONS OF THE VEIN OF GALEN
The natural history of aneurysmal malformations of the vein of Galen is now thoroughly understood and appropriate management of these disorders yields satisfactory results2. While about half the aneurysmal malformations of the vein of Galen are diagnosed before birth, the first clinical signs (systemic disorders) appear after birth and vary in their severity. Conditions range from heart failure with acute multi-organ failure and cerebromalacia that began in utero, to the accidental discovery of an enlarged cardiac silhouette with neither respiratory signs nor consequences on feeding. Clinical management depends on the severity of the clinical manifestations. Diagnosis is often easily made by auscultation of the skull, cranial ultrasound, CT scan or MRI. Angiography is not indicated for diagnostic purposes, as it will be performed with the first treatment. Lesions that are identified early on usually have a good prognosis: most infants who have aneurysmal malformations of the vein of Galen thrive until the age of 5 or 6 months, at which time endovascular treatment should begin regardless of the symptoms. Infants with heart failure require medical treatment (digitalis and diuretics or diuretics alone) until they can be embolized, which will also stabilize their hemodynamic status. More severe forms with intractable cardiac failure may require earlier or even immediate embolization of the main shunts. These infants may be identified using a neonatal score that we have developed over the last 10years. The object of urgent intervention is to disconnect at least 30% of the initial malformation so as to bring the infant to a clinically stable condition. A more complete treatment can be performed later with more technical ease and with less risk for subsequent neurocognitive impairment. Contraindications to treatment are a poor initial neurological score and evidence of brain damage on imaging, both a reflection on the severity of multiple organ failure (Fig. 12.2). Embolization, when it is well performed, produces satisfactory results in this type of disorder.
Aneurysmal malformations of vein of Galen cause venous hemodynamic disorders that induce macrocrania or ventriculomegaly (Fig. 12.4 and Fig. 12.5). The ventricular enlargement is not caused by compression of the aqueduct but by venous sinus congestion; later it may be secondary to atrophy of the brain. Although surgical ventricular drainage may temporarily solve the clinical problems due to intracranial hypertension it does not treat the venous hypertension. Transarterial endovascular treatment is the treatment of choice for it deals with venous hypertension, the basic cause of the lesion. Embolization must be performed before the symptoms become irreversible. Only by anticipating each stage in the natural history of the disease can severe consequences such as neurocognitive retardation, epileptic seizures and hemorrhage (secondary to occlusion of the jugular bulb and retrograde pial venous flow) be avoided. Embolization has its own complications. Stroke may occur from inadvertent embolization of arteries supplying functional tissue or iatrogenic compromise of the venous outlets.
MALFORMATIONS OF THE DURAL SINUS
Approximately one-third of dural sinus malformations are diagnosed in utero. They appear as a large sac that may already be thrombosed. This can rapidly cause diffuse bilateral cerebral venous ischemia as a result of venous hypertension. A consumption coagulopathy may also develop secondary to the large volume of coagulated blood within the sac. Moderate cardiac hypertrophy may develop later in infancy. Even when arteriovenous communications in the walls of the malformed sinuses do exist (Fig. 12.11), the prognosis depends on the alternative drainage for cerebral venous blood. An infant’s vascular system has not yet acquired the capacity to drain cerebral venous blood into the cavernous sinus, and consequently cortical veins are affected by thrombosis within the malformation. Thrombosis usually develops 2–3 months after birth, later if the sinus lesion is lateral. The use of anticoagulants is generally resorted to at this point, for it is more efficient than endovascular or surgical reconstruction of the sinuses and stabilizes the situation while allowing maturation, however late it may be, of the sinus. These malformations have a severe prognosis. Diagnosis is often made in the presence of macrocrania due to the size of the lesion. Cranial murmurs may not be heard on auscultation. Formal diagnosis is made with cranial ultrasound performed through the fontanel, and confirmed by the MRI and the CT scan.
CEREBRAL (PIAL) VASCULAR MALFORMATIONS (Fig. 12.10)
These are very rare in neonates and infants. They tend to present with clinical convulsions as a result of irritation or from ischemia of the cerebral veins on the cortex (Fig. 12.7). In infants, they are usually identified following a hemorrhage (Fig. 12.8), more often than by the presence of neurodevelopmental delay. Hemorrhage combined with suggestive findings on a CT scan and especially the MRI should lead to emergency endovascular treatment, even in neonates, to prevent focal brain ‘melting’. We maintain that although symptoms caused by aneurysmal malformation of vein of Galen often do not require immediate attention, rapid intervention is mandatory in confirmed true cerebral malformations (Fig. 12.6).




Fig. 12.7 Three-month-old baby presenting with seizures. T1 weighted MRI in sagittal views (a, b) shows an AVM (arrow, a) in the posterior part of the frontal lobe. Diffuse hemorrhagic infarcts (double arrows, a, b) are noted remote from the shunt. Angiography (c, d) demonstrated an AVM of fistulous type (arrowheads, c) draining into venous ectasias (asterisks, c). Poor hemispheric venous drainage was apparent because of the venous congestion in the pathological venous system and in the superior sagittal sinus (d).
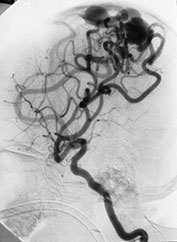

Fig. 12.8 A 3-day-old neonate presenting with seizures. A brain AVM was diagnosed at MRI (not shown) and confirmed at angiography (a). Embolization was planned but could not be performed because of intracranial hemorrhage. Analysis of the angio-architecture of the lesion after this hemorrhagic stroke (b) revealed an annexed venous false aneurysm (arrow) pointing to the exact rupture point of the AVM.


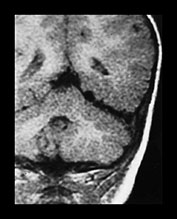
Fig. 12.9 A 20-month-old boy presenting with cardiac failure treated by digitalis and diuretics. Ultrasound of the brain detected a probable temporal AVM, confirmed by T1 weighted MRI in coronal (a) and axial (b) planes. Embolization was performed in order to counterbalance the effects of this high-flow AVM on the maturing brain. A repeat MRI (c) confirmed the subtotal occlusion of the AVM and the normal aspect of the brain parenchyma. Total occlusion of the lesion was obtained with a later procedure.
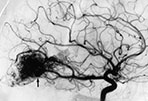


Fig. 12.10 Different architectural types of brain AVMs that can be encountered in the pediatric population: nidus type (a) with interposition of a pathological vascular network (arrow) between feeding arteries and draining veins; fistula type (b) with direct communication (arrow) between an enlarged artery and an ectatic vein; micro-AVM type (arrow, c) with normal-sized artery draining into a normal-sized vein.


Fig. 12.11 Dural sinus malformation diagnosed in an infant. T1 weighted MRI shows the giant venous lake corresponding to the malformed sinus (a), confirmed at angiography (b). Arteriovenous shunts into the malformation, fed by the middle meningeal artery (arrows) are demonstrated.
The growth and maturation of both the vascular system and brain at this age influence the management of vascular lesions. Indeed, whereas aneurysms and AVMs are relatively stable in adults, in children they are continually changing. Potential spontaneous remodeling, the extreme case being spontaneous thrombosis, or florid angiogenesis may not necessarily lead to rapid and total exclusion of the lesions. The current therapeutic objective is the minimum procedure that will ensure normal growth and maturation of the nervous system whilst preventing hemorrhage (Fig. 12.9). Experience shows that transarterial endovascular treatment is more flexible than surgery or radiotherapy and is associated with a low morbidity. Several procedures can be performed over a period of time and adjusted to the maturational stage of the brain.
ANEURYSMS
Very few aneurysms can be called ‘congenital’ apart from those that occur in fetal life due to inabilities to remodel the vascular system. This is illustrated by the small number of aneurysms diagnosed during infancy1, 2, 4, 5 Aneurysms are mainly classified according to their site.
‘Spontaneous’ aneurysms (non-traumatic) are divided into giant and non-giant aneurysms. An appearance of pseudodissection is often seen. A further category includes postinfectious and mycotic aneurysms, very rare occurrences in comparison with the prevalence of infection in the pediatric population. These aneurysms are often multiple. The type of infection that causes mycotic cerebral vascular lesions (meningitis, encephalitis or septicemia) is still unclear. Some multiple forms of aneurysm are linked to immune deficiencies transmitted in utero or acquired later in infancy, for example familial candidiasis, HIV infection.
When aneurysms are not found incidentally, the main presenting sign is intracranial hemorrhage and sometimes compression. The latter will cause a variety of symptoms depending on the site and size of the aneurysm. Focal neurological signs may be secondary to the volume of the lesion, but may also occur secondary to thrombosis or due to distal emboli sent out by the aneurysmal sac.
< prev | top | contents | next >
Conclusions
The technical management of congenital vascular disorders has been greatly modified over the past few years. The complete obliteration of lesions for ‘cosmetic’ purposes used to be the main objective. At times, its clinical toll was too high. Today, care and management of patients with vascular disorders is clearly multidisciplinary and involves neuropediatricians, interventional neuroradiologists and neurosurgeons who may, if need be, complete the endovascular treatment. Clinical follow-up examinations in a neuropediatric clinic are mandatory to assess the team’s previous decisions and measure results over the course of time. Although vascular lesions were thought to be rare several years back, they form an important part of Bicêtre’s activity (Table 12.4).
Techniques have become more precise because of the development of the finer, more supple and more hydrophilic microcatheters. Our objectives and the time required to reach them have been modified by a better understanding of the natural history and of the etiology of the disorders. Our therapeutic goal is to allow the child’s normal neurological development and normal brain maturation whilst minimizing the risk of complications from the lesion, for example hemorrhage.
< prev | top | contents | next >
Table 12.4 Breakdown of vascular diseases managed per age group (458 cases <16 years) Bicêtre 1985–1998
| Vein of Galen aneurysmal malformation (n = 213) | Neonates 95 | Infants 83 | Children 35 |
| Dural sinus malformation and arteriovenous shunts (n = 29) | Neonates 8 | Infants 14 | Children 7 |
| Pial arteriovenous shunts (n = 197) | Neonates 7 | Infants 25 | Children 165 |
| Arterial aneurysms (n = 19) | – | Infants 4 | Children 15 |
Summary
- ‘Congenital vascular malformation’ is a biological concept rather than a structural one. Malformations can be present and detected in utero, but in most instances they appear to be ‘triggered’ and revealed postnatally.
- Two lesions may be diagnosed in utero: the vein of Galen aneurysmal malformation and dural sinus malformation.
- Cerebral arteriovenous malformations and arterial aneurysms are diagnosed much later during life.
- During the first 2 years of life there is significant cardiovascular maturation, as well as cerebral venous and dural sinus maturation. The brain is very vulnerable to ischemic injury during this period. Management of any malformation must allow for satisfactory brain maturation.
- Brain damage from vascular malformations during this period usually arises from ischemia secondary to venous congestion or thrombosis.
- Ventriculomegaly is primarily caused by venous congestion, but it may occur later as a result of cerebral atrophy. In some cases an active hydrocephalus may develop.
- Treatment of these lesions is optimally by a transarterial endovascular approach at all stages of the diseases.
- Familial vascular diseases are rarely diagnosed in the perinatal period. In particular, familial diseases giving aneurysms lead to arterial problems in adulthood, which most likely require a secondary mutation to reveal the underlying genetic defect.
< prev | top | contents | next >
References
- Edwards M and Hoffman H (1989) Cerebral Vascular Disease in Children and Adolescents. Baltimore, Williams & Wilkins.
- Lasjaunias P (1997) Vascular Disease in Neonates, Infants and Children. Interventional Neuroradiology Management. Berlin, Springer.
- Levene M, Lilford R, Bennett M et al. (1995) Fetal andNeonatal Neurology and Neurosurgery. Edinburgh, Churchill Livingstone.
- Raimondi A, Choux M and di Rocco C (1991) Cerebrovascular Diseases in Children. New York, Springer.
- Roach ES and Riela AR (1995) Pediatric Cerebrovascular Disorders. New York, Futura.