Imaging the preterm infant:
practical issues
Elia F Maalouf and Serena J Counsell
Chapter Contents
- Introduction
- The neonatal scanner
- Transport of the baby
- Ventilation
- Immobilization
- Temperature maintenance
- Sedation
- Monitoring
- Pulse sequences and scanning parameters
- Safety issues
- Summary
- References
Introduction
Magnetic resonance imaging (MRI) is an established method for determining the presence and extent of lesions in adults and older infants. However, very few studies have been performed on preterm infants and these have only been undertaken on infants of 29 weeks of gestational age (GA) or more 3, 10, 13, 14 . This is primarily because of difficulties in transporting, temperature maintenance, continuing intensive care and monitoring preterm infants. In order to overcome these problems, a purpose-designed 1.0T system (Oxford Magnet Technology Ltd, Eynsham, Oxford, UK and Marconi (Picker) International Inc., Cleveland, Ohio, USA) has been installed in our neonatal intensive care unit (NICU)5. Using this system it is possible to provide the same standard of care for the infants in the scanner as they receive elsewhere in the NICU. As a result it has been possible to study very premature neonates as young as 24 weeks GA 1, 2, 9, .
< prev | top | contents | next >
The neonatal scanner
The integration of an MR scanner into an NICU provides a unique opportunity to image preterm infants and infants in the acute phase of an insult, without compromising their intensive care. The neonatal MR scanner was designed to provide access to the infants at all times whilst they were being scanned. In order to achieve this, the length of the magnet bore was made very short (38cm) (Fig. 2.1). Active shielding is employed within the magnet but paradoxically this results in a 2T fringe field at a radius of 50cm from the center of the bore of the 1T magnet. The 5-Gauss line is at a distance of 3m from the center of the bore. The maximum gradient strength is 40mT/m in all three axes with a maximum gradient slew rate of 40mT/m/ms. A specially designed transmit/receive quadrature birdcage coil (32cm x 23cm) is used for all examinations and allows infants to be studied up to a maximum weight of about 5kg. The short Z axis of the magnet results in a discoid volume of useful homogenous field (≤10ppm) (14cm in the X direction, 14cm in the Y direction and 4cm in the Z direction).
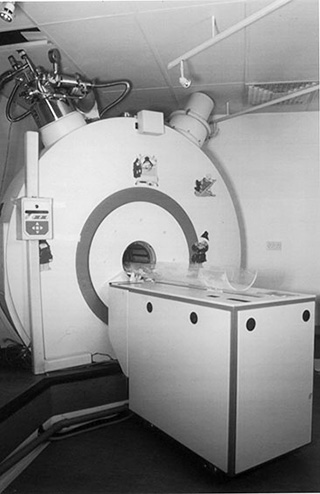
Fig. 2.1 The 1.0 Tesla neonatal scanner (Oxford Magnet Technology, Oxford and Marconi (Picker) International Inc., Cleveland Ohio).
< prev | top | contents | next >
Transport of the baby
A specially designed non-magnetic transport trolley has been made of similar dimensions to the incubators used on the ward. The main design objective of the transport trolley was to minimize handling of the baby whilst being able to continue life monitoring, and ventilation where required, during the move from incubator to the imaging system.
The baby is placed on a specially made perspex cradle, which fits into the transport trolley (Fig. 2.2). Using this system, infants are only handled when being moved from their cot or incubator to the trolley and back to their cot from the trolley after scanning. This cradle has a fastening device at one end for securing ventilator tubing and infusion lines. Infusion lines are extended and all infusion pumps and monitoring equipment are placed on shelves on the transport trolley.
As monitoring equipment used on the NICU is not MR compatible, all monitoring is switched to MR-compatible monitoring and the oxygen line is attached to the piped supply at the door of the scanning room. Infusion pumps are attached to a wall-mounted rail within the scanning room but beyond the 5-Gauss line. The perspex cradle is transferred from the trolley to a sliding, dished, perspex bed on the scanner table.
< prev | top | contents | next >
Ventilation
Ventilated infants may be imaged using an MR-compatible ventilator (babyPAC neonatal, pneuPac Limited, UK) (Fig. 2.3), which can be sited adjacent to the scanner.
If an MR-compatible ventilator is not available, ventilation may be performed using a ventilator sited in a radio frequency (RF) cupboard, with ports for the ventilator tubing, or by securing the ventilator outside the 5-Gauss line. If these methods are used, ventilator tubing will have to be extended and the positive inspiratory pressure, the end expiratory pressure valve and the gas flow must be adjusted to allow for the ‘dead space’ of the tubing.
< prev | top | contents | next >
Immobilization
Effective immobilization of the neonate is vital in order to obtain high-quality MR images. Immobilization is achieved with an Olympic bag, which contains small polystyrene balls and is evacuated with suction to fit snugly around the baby’s head. The Olympic bag also helps to muffle sound. Babies who are not ventilated are imaged on their side whenever possible but as a large number of babies are not sedated for imaging, it is most important to find a position that is comfortable for the baby and which can be maintained for the duration of the scan. Ventilated babies need to be imaged supine to accommodate the endo- or nasotracheal tube and ventilator tubing.
< prev | top | contents | next >
Temperature maintenance
In order to maintain the infant’s temperature, he or she is swaddled in blankets and the temperature of the scanning room is set at 27°C, the same as the temperature on the neonatal unit. Additional heating for extremely preterm neonates can be provided with bubble wrap, woollen hats and ‘gel bags’, which retain their heat once warmed in a microwave oven.
< prev | top | contents | next >
Sedation
Sedation with oral chloral hydrate (20–30mg/kg) is occasionally necessary but the majority of preterm infants and neonates can be successfully imaged following a feed or whilst sedated for ventilation4.
< prev | top | contents | next >
Monitoring
Physiological data must be recorded throughout an MRI examination when imaging preterm infants. We use a Hewlett Packard Merlin life support system. All monitoring equipment for use in the scanner room must either be MR compatible or be RF shielded. In our case the monitor is sited in an RF-shielded cupboard within the scanning room. Oxygen saturation, heart rate, temperature and, where required, mean arterial blood pressure are monitored throughout the examination.
Oxygen saturation can be measured by pulse oximetry (Nellcor oxiband or oxisensor D-20 transducer with a Nellcor pulse oximeter, Nellcor Incorporated, Pleasanton, CA, USA) and heart rate may be measured using chest ECG leads. ECG leads and electrodes must be MR compatible and we use Blue sensor (Medicotest, Olstykke, Denmark) ECG electrodes and ECG leads with current limiting resistors (NDM Division, American Hospital Supply Corporation, Ohio, USA). RF fields can cause currents in conduction loops, which may cause burns7 and, therefore, care must be taken to ensure that ECG leads do not form conductive loops and skin contact is kept to a minimum. The electrodes should be placed close together, but not touching, to minimize ECG interference by the magnetic field and ECG leads should be plaited in order to minimize loops across which potential differences may occur11. Temperature may be measured by placing an MR-compatible temperature probe in the axilla. In the neonatal scanner, all monitoring devices are connected via a gantry, which prevents loose cables stretching across the floor and causing a potential hazard.
As a safety precaution, an experienced pediatrician remains in the scanning room with the infant during scanning and can view the monitor through the glass window of the RF-shielded cupboard. A further monitor is sited in the scanner control room.
< prev | top | contents | next >
Pulse sequences and scanning parameters
The neonatal brain has a higher water content (92–95%) than the adult brain (82–85%) and so T1 and T2 values are greater6. This means that echo times (TE), repetition times (TR) and inversion times (TI) have to be increased. Transverse T1 weighted conventional spin echo (CSE), T2 weighted fast spin echo (FSE) and inversion recovery fast spin echo (IRFSE) images are obtained in each study and in some cases coronal and sagittal T2 weighted FSE images are obtained. T1 weighted CSE images have low gray/white matter contrast but are useful in assessing hemorrhage, brain swelling in hypoxic ischemic encephalopathy and for assessing tissue enhancement after administration of contrast. The T2 weighted FSE sequence is useful to demonstrate pathology and fast imaging techniques are helpful to reduce motion artifact in neonatal imaging. We have found the T2 weighted FSE to be the optimal sequence for demonstrating myelination in the premature brain. The IRFSE sequence provides excellent gray/ white matter contrast and is useful in assessing myelination.
Diffusion weighted and angiographic sequences are performed where indicated and T1 weighted sequences are obtained after intravenous gadolinium (0.1mmol/kg gadopentate dimeglumine) in selected cases.
Sequence parameters used on the neonatal scanner are listed in Table 2.1.
< prev | top | contents | next >
Safety issues
The number of people in the scanning room should be kept to a minimum but in the case of ventilated babies two pediatricians and a radiographer are essential. We exclude parents from the scanning room but they are welcome to watch from the control room. A metal check form is completed by the pediatrician or nurse caring for the baby and checked by the radiographer before transporting the baby to the magnet. This form includes specific neonatal items such as Serle arterial lines with terminal electrodes, electronic name tags and metal poppers on clothes (see Chapter 1). The door of the neonatal scanning room is kept locked by a magnetic latch system with one push-release button being in the control room and one inside the scanning room.
Emergency resuscitation equipment should be kept either in the scanning room or in the immediate vicinity. Any equipment kept within the scanning room must be MR compatible and so, in many centers, resuscitation equipment is kept just outside of the scanner room. If this is the case, the infant must be brought out of the scanning room for emergency treatment to avoid incompatible equipment being brought into the MR environment. In the neonatal scanner at the Hammersmith Hospital, we keep an MR-compatible laryngoscope, stethoscope, endotrachial (ET) tubes and introducers and ET suction equipment within the scanner room. If the infant requires anything further they are returned to the NICU for treatment.
Acoustic noise is produced through the vibration of the gradient coils as electric current through the coils is switched on and off. Acoustic noise exposure has been reported to cause increased stimulation in neonates8. The neonatal scanner at the Hammersmith Hospital has several noise reduction measures incorporated into the design, including lagging and gradient cable immobilization. To reduce acoustic noise levels still further, an Olympic bag filled with polystyrene balls is evacuated of air and is used as immobilization. As the immobilization bag fits snugly around the infant’s head, it is an effective method of reducing the infant’s exposure to acoustic noise. Acoustic noise levels in the neonatal scanner during different pulse sequences were measured and found to be below the recommended safe limits and are described in Table 2.2.
One study has reported physiological changes in infants undergoing MRI, including raised heart rate, BP or desaturation, probably due to stimulation whilst undergoing MRI12. However, in a recent study infants did not show an excessive disturbance in heart rate whilst undergoing MRI1. A slight increase in heart rate and temperature was observed towards the end of the scan but these increases were not clinically significant. This study concluded that there have been no detrimental physiological effects on infants undergoing MRI in the neonatal scanner1.
Table 2.1 Pulse sequences parameters
| Pulse sequence | TR (ms) |
TI (ms) |
TE (ms) |
Slice thick (mm) |
Slice gap (mm) |
No. of slices |
NSA | Phase matrix |
Scan time (mins) |
Echo train length |
|
|---|---|---|---|---|---|---|---|---|---|---|---|
| T1 weighted CSE | 600 | – | 20 | 4 | – | 9 | 2 | 192 | 3:30 | – | |
| T2 weighted FSE | 3500 | – | 208 | 4 | – | 9 | 4 | 256 | 3:50 | 16 | |
| IRFSE | 3697 | 950 | 36 | 4 | 1 | 6 | 4 | 256 | 3:45 | 16 | |
| CSE, conventional spin echo; FSE, fast spin echo; IRFSE, inversion recovery fast spin echo; NSA, number of signal averages. | |||||||||||
Table 2.2 Acoustic noise measurements
| Pulse sequence | MAXL (dBA) | Leq (dBA) |
|---|---|---|
| T1 weighted CSE | 70 | 67 |
| T2 weighted FSE | 72 | 70 |
| IRFSE | 71 | 67 |
| Open incubator | 62 | – |
| Transport incubator with alarm and compressor | 66 | – |
| MAXL, maximum root mean squared level; Leq, average sound pressure level during the
measurement period; dBA, a weighted decibel. | ||
< prev | top | contents | next >
Summary
- MRI of preterm infants receiving intensive care can be safely performed using a dedicated neonatal scanner situated on a neonatal intensive care unit.
- Excellent image quality can be produced once adaptations to coils for the small head and sequences for the immature brain have been made.
- Meticulous attention to detail has to be made in transferring a sick ventilated infant into the scanner. Two neonatally qualified staff are needed in addition to the radiographer.
- All intensive care monitoring equipment must be MR compatible.
- Temperature maintenance may be achieved by controlling the room temperature and the immediate environment of the infant.
- Some imaging sequences may be unacceptably noisy and noise reduction measures must be made.
- Fast imaging sequences are ideal to decrease the examination time and to avoid the unnecessary use of sedation although some image detail may be sacrificed.
< prev | top | contents | next >
References
- Battin M, Maalouf EF, Counsell SJ et al. (1998a) Physiological stability of preterm infants during magnetic resonance imaging. Early Human Development 52, 101–110.
- Battin MR, Maalouf EF, Counsell SJ et al. (1998b) Magnetic resonance imaging of the brain in very preterm infants: visualization of the germinal matrix, early myelination, and cortical folding. Pediatrics 101, 957–962.
- Childs A, Ramenghi LA, Evans DJ et al. (1998) MR features of developing periventricular white matter in preterm infants: evidence of glial cell migration. Am J Neuroradiol 19, 971–976.
- Cowan FM (1997) Sedation for magnetic resonance scanning for infants and young children. In: Whitman JG and McCloy R (Eds) Principles and Practice of Sedation. London, Blackwell Healthcare, pp. 206–213.
- Hall AS, Young IR, Davies FJ et al. (1997) A dedicated magnetic resonance system in a neonatal intensive therapy unit. In: Bradley WG and Bydder GM (Eds) Advanced MR Imaging Techniques. London, Martin Dunitz, pp. 281–290.
- Johnson MA, Pennock JM, Bydder GM et al. (1983) Clinical NMR imaging of the brain in children: normal and neurologic disease. Am J Roentgenol 141(5), 1005–1018.
- Kanal E and Shellock FG (1990) Burns associated with clinical MR examinations. Radiology 175, 585.
- Long JG, Lucey JF and Philip AG (1980) Noise and hypoxaemia in the intensive care nursery. Pediatrics 65, 143–145.
- Maalouf EF, Duggan PJ, Rutherford MA et al. (1999) Magnetic resonance imaging of the brain in a cohort of extremely preterm infants. J Pediatr 135, 351–357.
- McArdle CB, Richardson CJ, Nicholas DA et al. (1987) Developmental features of the neonatal brain: MR imaging. Part 1 gray–white matter differentiation and myelination. Pediatr Radiol 162(1), 223–229.
- Peden CJ, Menon DK, Hall AS et al. (1992) Magnetic resonance for the anaesthetist. Part 2: anaesthesia and monitoring in MR units. Anaesthesia 47, 508–517.
- Philbin MK, Taber KH and Hayman LA (1996) Preliminary report: changes in vital signs of term newborns during MR. Am J Neuroradiol 17, 1033–1036.
- Sie LT, van der Knapp MS, van Wezel-Meijler G et al. (1997)MRI assessment of myelination of motor and sensory pathwaysin the brain of preterm and term-born infants. Neuropediatrics 28(2), 97–105.
- Van der Knapp MS, Barkhof F, Ader HJ et al. (1996) Normal gyration and sulcation in preterm and term neonates: appearances on MR images. Radiology 200, 389–396.

